High Pressure Cold Processing

HPCP Food Safety
High Pressure Cold Processing
Human and Pet Food Safety The Natural Way
This Information Is presented by: Morasch Meats Inc /PressureSafe™ LLC- Wood Village, Oregon (MMI/PSI)
History of HPCP

- 1896 to 1914 Professor at West Virginia Agricultural Experiment Station study the effect of hydrostatic pressures on the inactivation of microbes in fruits, vegetables and milk.
- 1982 to 1988 Professors at the University of Delaware prove that pressures of 50,000 pounds per square inch (psi) can inactivate a wide range of pathogenic and food spoilage microbes.
- 1988 to 1993 The Japanese food industry forms a research consortium to commercialize high pressure food preservation. The first products are marketed in Japan using pressures of 60,000 psi.
- 1993 to 1995 The US Army initiates a study of food preservation by high pressure to obtain better quality rations.
- 1997 – Avomex, Inc, is formed to pressure treat avocado puree (guacamole) and the first commercially recognized HPP product is sold in the US marketplace.
 What is HPCP
What is HPCP
- There are 3 components to the technology. Pressure (as high as 87,000 psi or 6,000 bars). Hold time (generally 2 to 3 minutes) and Chilled Water Temperature.
- High pressure cold processing consists of applying ultra high water pressure for a short period of time, to a packaged food product. The packaged food is loaded into a basket and conveyed into the vessel. The vessel is sealed and then pressurized with chilled water. until the defined pressure is reached.
Pressure Safe LLC
 A Family owned business, Established in 1956, Morasch Meats has always been recognized as having the highest quality and safety standards in the meat manufacturing industry. To insure the success and continuing quality and safety for generations to come, the Morasch family invests in education, technology and innovation to insure that the highest quality and most rigerous standards are maintained.
A Family owned business, Established in 1956, Morasch Meats has always been recognized as having the highest quality and safety standards in the meat manufacturing industry. To insure the success and continuing quality and safety for generations to come, the Morasch family invests in education, technology and innovation to insure that the highest quality and most rigerous standards are maintained.
How HPCP Works
High pressure cold processing systems employ pressures to inactivate many food-borne pathogens such as Salmonella, E. coli and Listeria, with no change in organoleptic properties or nutritional value. HPCP works because uniform high pressure affects microbial cellular integrity and metabolism without affecting the covalent structures of food components responsible for nutrition (structural proteins, fibers, fats, enzymes etc.). Once the cell wall is fractured, within 24 to 36 hours, the natural acidic properties of the product then inactivate the pathogens. Specific pressure and hold times are carefully monitored to ensure HPP does not affect the good bacteria. Molecular bonds are not broken during HPP, and as a result, no free radicals or chemical by-products are formed. The duration of the Pressurization phase (generally 2 to 3 minutes) determines the efficacy of pathogen inactivation. The HPP process does not affect the structural integrity of the package used, as the pressure is simultaneously applied uniformly on the food and package.
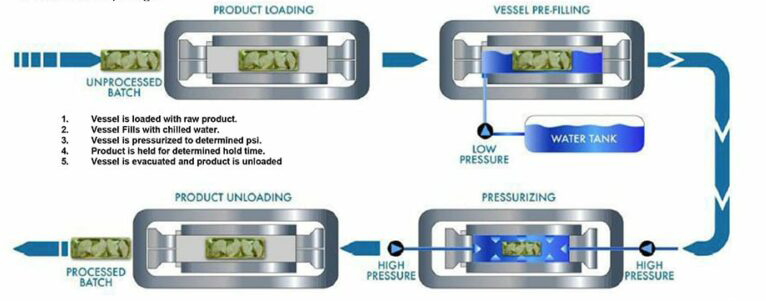

Lets Look at the Process
These are actual photos of non-HPCP chubs and HPO chubs for side-by-side comparison. Using our state-of-the-art equipment, there is no difference in color or texture. Other companies may use inferior ingredients, older – less accurate processors, or improper care during the HPCP process, which can result in rubbery texture, color change, and sticking to chub film. We do not see any of these concerns with any of the brands and products we produce using our HPCP apparatus. While other processors may use different equipment, pressure, and hold times, and not use chilled water, MMI/PSI consistently combines the correct factors when using HPCP. This is absolutely crucial for producing true raw food. With ongoing research, education, and testing, MMI/PSI can assure you that all products from our facility will have the highest quality, bio-available nutrition available.
HPCP Advantages
- Offers manufacturers of raw food diets/products a non-thermal process to insure pathogen inactivation.
- 5 log reduction achieved at 87,000 psi inactivating pathogens – Salmonella, E. coli, and Listeria.
- Eliminates the need for chemical additives, irradiation, heat-applied methods (steam), or additives of any kind.
- Attractive for consumers – Meets the demand for safe raw pet food products through non-thermal processing.
- Permits the inactivation of microorganisms at low temperatures, while valuable nutritional components such as protein, fats, vitamins, minerals, enzymes, and beneficial bacteria remain unaffected.
Foods Suitable for HPCP
Low-medium moisture, semi-solid/solid foods, vacuum packaged:
- Dry-cured or cooked meat products

- RTE (ready-to-eat) Foods
- Cheeses
- Fish, seafood, marinated products
- Ready-to-eat meals, sauces
High moisture, liquid foods in plastic bottle/flexible packaging:

- Dairy products
- Fruit juices – many fresh juices with no additives have been HPP’d with no compromise in the taste.
- Bioactive beverages
- Baby food
High moisture, solid foods, vacuum packaged:

- Raw meat and poultry products
- Fresh fruits and vegetables
NWN Raw Pet Food Feeding Trial

We realize that there are many opinions, myths, and misconceptions regarding the HPP process. This often stems from a lack of available information on the process Itself and the effects of HPP on raw, frozen products for your pets. In an effort to understand the process better, NWN funded a year-long study that provided tangible data on the subject. This study was devised and monitored by an independent veterinarian who was not a proponent of raw diets for pets.
Our veterinarian and his assistants followed a year-long feeding trial with 100 Alaskan Huskies, all active, working dogs. 8 dogs varying In age and sex were selected as representative of the whole kennel. These dogs were closely monitored with blood analysis, complete physical exams, and fecal analysis at the beginning of the trial, the end of the trial, and at each intermediate stage of the trial.
The first 26-week portion of the feeding trial was conducted by feeding non-HPP Northwest Natural Raw Turkey Dinner for Dogs. The dogs were examined and tested at the beginning of the trial, at 14 weeks, and then again at 26 weeks. The second 26-week portion of the feeding trial was conducted by feeding the HPP Northwest Naturals Turkey Dinner for Dogs. The same criteria of exams and tests were administered at the beginning of the trial, at 14 weeks, and with final testing done at 26 weeks.
Test results:- no changes in bloodwork levels while the dogs were on non-HPP or HPP products. All fecals were negative for salmonella. Improved differences were seen in skin, coat and the overall condition of the dogs at the end of the trial.
Our team of experts have worked closely with leading authorities on HPCP processing and have developed proper techniques to keep the nutritional values of all HPCP’d product at the highest levels possible. If you would like specifics on how we conduct and monitor the HPCP process in our facility, please inquire with your national sales representative. You may also view a video of our In-house HPCP process to see product prior to forming that is run through our own HPCP machine, showing that product color, texture, and temperature are not altered. You will clearly see that HPCP product is not cooked and the product is kept chilled and cool at all times. Please contact us with any questions or concerns.
Our Quality Ingredients
 USDA (U.S. Department of Agriculture) inspected and passed meats, poultry and game passed for human consumption.
USDA (U.S. Department of Agriculture) inspected and passed meats, poultry and game passed for human consumption.- FDA/GRAS inspected fruits and vegetables passed for human consumption.
- Incorporation of natural vitamins, proteinated minerals and essential fatty acids.
- All products produced in the USA following FDA and USDA guidelines for safe food handling and production.
Our Food Safety
- Products produced using HPP cold water process.
- Positive test release procedures using certified testing, verifying absence of Salmonella, E. coli and Listeria on all batches, all products prior to shipping
- Products produced and regulated daily in USDA/FDA human food facilities or State Department of Agriculture facilities in accordance with all applicable USDA and FDA human food safety and sanitation regulations.
- Strict HACCP* programs in place to insure product safety, proper chain of custody and cold chain standards.
*(Hazard Analysis Critical Control Points) - Pre-Operational and Post-Operational plant inspections with bioluminescence swab testing of all contact surfaces and equipment to insure work environment is free of pathogens.
- Random testing of incoming raw materials using SDIX/AOAC Certified testing (meat, poultry, game and produce) for the presence of Salmonella, E.coli and Listeria.
- USDA Inspector on site during all production.
- Annual 3rd Party Audits -Always scoring in the highest level.
The Importance of 3rd Party Audits
Northwest Naturals, Morasch Meats and Pressure Safe all participate in third party audits. Annually, we use the prestigious BRC Audit within the top of the standard and have passed every year.
3rd party food safety audits are an additional objective review of all the food safety programs and standards in place at a given facility. These audits are performed by experienced professionals, usually doctors, who spend several days in a facility observing and reviewing production, programs, records and all facility details. These professional auditors are able to compare each plant against all other plants that they inspect worldwide. Their reports are written at great length, detail and cost.
The BRC Global Standards is a leading food safety and quality certification program, used by over 23,000 certified suppliers in one hundred and 23 countries. It is now a fundamental requirement of leading retailers all over the world. This certification is issued through a worldwide network of accredited certification bodies. The Standards guarantee the standardization of quality, safety and operational criteria of all products produced in our facility. It ensures that manufacturers fulfill their obligations and provide protection for the end consumer – you and your pet.
Most pet food production in the United States operates under the following food safety regulatory programs:
FDA (Food & Drug Administration) regulated facilities or in facilities inspected by State Department of Agriculture. FDA is seldom in a facility and may show up once every 10 to 15 years or when a food recall happens for food safety reason.s. State Department of Agriculture is a regulatory group that oversees a small percentage of pet food facilities. Inspections are minimal and infrequent. Typically inspectors show up once every few years for a cursory overview of food production.
Very few pet food companies use third party audits to review and Insure their safety standards, especially the eminent BRC. But in our opinion, third party audits are an essential step in food safety, especially in the pet food industry where products from foreign countries and lower food safety practices have resulted in numerous food safety recalls and sick or dying pets. Pet food companies such as Northwest Naturals, that pass third party safety audits and are processed in an USDA regulated facility should be important considerations in your decision on which brands or products to feed your pets or supply to customers. Not all pet food production takes place with the same standards or controls. Products from many foreign countries have resulted In numerous food safety recalls due to lower food safety standards.


All Northwest Naturals Raw Frozen and Freeze Dried Products produced at MMI/PSI are manufactured under the following USDA Human Food Safety Guidelines.
- HACCP Hazard Analysis Critical Control Points
- GMP Good Manufacturing Practices
- TQC Total Quality Control
- SSOP Standard Sanitation Operating Procedures
- PREOP Preoperational Inspections
- PIP Planned Improvement Program
Always scoring among the highest scores in Annual 3rd Party Food Safety Audits Manufactured in a facility achieving Certification under the BRC – Global Food Safety Initiative Oregon Tilth APHIS Approved Facility (Animal and Plant Health Inspection Service).
Why Food Safety
MMI/PSI have a very strong focus on the balance between nutritional integrity and the safety of our finished products. We emphasize the quality of ingredients and food manufacturing safety standards it takes to produce the highest quality Raw Pet Foods that retain the highest standard of bio-available nutrients. Our products are never subjected to chemicals, steaming, irradiation or heat.
Always fresh, Always the best!
The Food Safety Modernization Act of 2010 (H.R. 2751)
The Food Safety Modernization Act of 2010 (H.R. 27Sl) was signed into law by President Obama on January 4, 2011. It aims to ensure the U.S. food supply is safe by shifting the focus of federal regulators from responding to contamination to preventing it. The Food Safety Modernization Act (FSMA) has given the Food and Drug Administration (FDA) new authorities to regulate the way foods are grown, harvested and processed. The law grants FDA a number of new powers, including mandatory recall authority, which the agency has sought for many years. The FSMA requires FDA to undertake more than a dozen rule makings and issue at least 10 guidance documents, as well as a host of reports, plans, strategies, standards, notices and other tasks.
Summary of HPCP Benefits
- Inactivates food-borne pathogens such as Salmonella, E. coli and Listeria, with no change in organoleptic properties or nutritional value.
- Non-Thermal process, does not alter protein structure, nutrients or enzymes.
- Eliminates the need for radiation, chemical preservatives or additives of any kind.
- Offers consumers safe and nutritious raw food products for people and their pets without compromising product integrity.
- Does not alter the palatability of ingredients.
The Correct Answers to Common Misconceptions About High Pressure Processing Cont.
A great quote:
“Words being twisted, combined with lack of information and provided to consumers results in misleading information.”
Misconceptions
Misconception: HPCP does not kill the spores that may form when bacteria go into hibernation mode.
Truth: With science and testing, existing bacteria that is inactivated by the natural acidic properties in the recipe are dead. Current shelf life ls extended.
Misconception: After HPCP meat is re-ground due to cooked appearance.
Truth: Since there is no heat involved, the product is not cooked. The regrinding is for forming recipes into nuggets, bars, and nibbles.
Misconception: HPCP is used because manufacturer uses inferior denatured meats
Truth: NWN used only the highest grade, USDA inspected and passed human edible meats and all produce is the same produce you buy in the grocery store – in our recipes.
Misconception: HPCP destroys beneficial bacteria, leaving harmful spores to freely proliferate to infectious levels.
Truth: With the correct PSI and dwell time, only the bad pathogens are inactivated. Good bacteria is left intact.
Misconception: Pasteurization depletes and irreversibly denatures enzymes, vitamins, essential fatty acids, and beneficial bacteria.
Truth: In the classic sense of the word “pasteurization” has always involved heat, and high temperatures can indeed affect nutrients. But – in the 21st century, we have the new technology of cold pasteurization, which combines very high pressure with very cold temperatures. HPP uses no heat to process products; hence there is no depletion or denaturing of enzymes, vitamins, essential fatty adds, or beneficial bacteria. Correct pressure and dwell time is calculated to only inactivate Salmonella, E. coli, and Listeria.
Misconception: Processing deteriorates the molecular composition of meat.
Truth: Processed meat is considered to be any meat which has been modified in order either to improve its taste or to extend its shelf life. Methods of meat processing include salting, curing, fermentation, and smoking. However, meats that have undergone simple mechanical processes such as cutting, grinding or mixing are not classified as processed meats”.






